潘勤,李定国,汪余勤,徐芹芳,上海第二医科大学附属新华医院消化内科 上海市200092
潘勤,男,1971-03-12生,上海市人,汉族.1994年上海铁道医学院本科毕业,2000年上海第二医科大学硕士研究生毕业,现为上海第二医科大学博士研究生.主要从事肝纤维化的发生机制及药物防治研究.
项目负责人 潘勤,200092,上海市控江路1665号,上海第二医科大学附属新华医院. fangchunhua@online.sh.cn
电话:021-65790000-5319 传真:021-55296348
收稿日期 2002-05-02 接受日期 2002-06-10
Effects of somatostatin on activated rat hepatic stellate cells and its mechanisms
Qin Pan, Ding-Guo Li, Yue-Qin Wang, Qin-Fang Xue
Qin Pan, Ding-Guo Li, Yue-Qin Wang, Qin-Fang Xue, Department of Gastroenterology, Xin Hua Hospital, Shanghai Second Medical University, Shanghai 200092, China
Correspondence to: Qin Pan, Xin Hua Hospital, 1665 KongJiang Road, Shanghai 200092, China. fangchunhua@online.sh.cn
Received 2002-05-02 Accepted 2002-06-10
Abstract
AIM:To investigate the effects of somatostatin on proliferation and apoptosis of activated rat hepatic stellate cells (HSCs).
METHODS:Being isolated from liver of SD rat by in situ perfusion with collagenase and pronase and density gradient, HSCs became activated after 10 days. Then the rates of apoptosis and proliferation of HSCs, which had been treated with different dosage of somatostatin, were measured by acridine orange/ethidium bromide fluorescent stain, terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling, transmission electron microscopy, flow cytometry and MTT assay. Furthermore, the mechanisms of somatostatin were investigated with the aid of cytodynamic analysis.
RESULTS:somatostatin was able to decrease the proliferation rate as well as promote the programmed cell death of activated rat HSCs.Its dose-dependent action,in which G0/G1 arrest played an important role, was strongest when the concentration of somatostatin reached 10-6mol/L or 10-7mol/L.
CONCLUSION:Anti-proliferative and proapoptotic effects on activated rat HSCs can be obtained by a low dose of somatostatin. These findings reveal the potential antifibrotic value of somatostatin.
Pan Q, Li DG, Wang YQ, Xue QF. Effects of somatostatin on activated rat hepatic stellate cells and its mechanisms. Shijie Huaren Xiaohua Zazhi 2002;10(11): 1250-1252
摘要
目的:研究生长抑素(somatostatin,SST)与大鼠肝星状细胞(hepatic stellate cell,HSC)增生、凋亡的关系.
方法:分离、培养的原代大鼠HSC,经不同浓度SST (10-6-10-10 mol/L)处理72 h后,以吖啶橙(acridine orange,AO)/溴乙啶(ethidium bromide,EB)荧光染色法、末端核苷酸转移酶介导的脱氧三磷酸尿苷原位缺口末端标记法(terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling,TUNEL)、透射电镜及流式细胞术检测其凋亡率的改变.SST作用120 h后,以甲基噻唑基四唑(methyl thiazolyl tetrazolium,MTT)比色法检测HSC增生率的变化.并通过对细胞动力学的观察,探讨SST的作用机制.
结果:SST可通过剂量依赖方式抑制HSC增生,提高HSC凋亡率.浓度为10-6 mol/L或10-7 mol/L时效果尤为显著.G0/G1期阻滞是SST发挥作用的重要途径.
结论:低剂量SST即可抑制HSC增生,并促进其凋亡,提示SST在抗肝纤维化方面具有潜在价值.
潘勤,李定国,汪余勤,徐芹芳.生长抑素对肝星状细胞的影响机制. 世界华人消化杂志 2002;10(11):1250-1252
0 引言
近年来,生长抑素(somatostatin,SST)在治疗肝硬化所致的上消化道出血中应用日益普遍[1].同时,由于SST对细胞增生及EGF、TGF-a等促增值细胞因子的分泌具有广泛抑制作用,可能影响肝硬化形成的关键细胞-肝星状细胞(hepatic stellate cell,HSC)乃至肝硬化本身.故我们尝试以不同浓度SST处理原代大鼠HSC,籍此了解SST对HSC增生、凋亡的影响及其具体机制.
1 材料和方法
1.1 材料 ♂SD大鼠,质量250-300 g(中国科学院上海实验动物中心);SST(Serono公司),DMEM培养基(Gibco公司),MTT(Sigma公司),AO,EB(上海化学试剂采购供应站),TUNEL试剂盒(武汉博士德公司);960型全自动酶标仪、Olympus IC50型荧光显微镜、FACS420型流式细胞仪、Hitachi H-500型透射电子显微镜.
1.2方法 大鼠HSC分离采用改良Friedman法[2,3],经门静脉依次用无Ca2+灌流液、链霉蛋白酶溶液、胶原酶溶液灌流,然后剪碎肝脏,置于含链霉蛋白酶、胶原酶及DNA酶的溶液中震荡30 min.细胞悬液离心后洗3次,再加入Nycodenz离心.吸取表面的HSC,重悬于DMEM培养液中.台盼兰染色显示细胞存活率达90 %以上,波长328 nm紫外线激发下90 %以上细胞发出蓝色荧光.培养10 d后95 %以上HSCa-SMA染色阳性.
1.2.1 HSC增生检测 将HSC接种于96孔培养板(约1×104/孔),分为对照组及5个浓度SST处理组(6复孔/组).细胞贴壁后加入SST,使各组终浓度分别为0,10-6,10-7,10-8,10-9及10-1 0mol/L.培养5 d后再加MTT溶液,继续孵育4 h.弃上清液,加DMSO,于490 nm波长处测出各孔吸光度(A值),按下式计算:细胞抑制率=(1-给药孔平均A值/对照孔平均A值)×100 %.
1.2.2 HSC凋亡及细胞周期检测 按同样分组方法,将HSC接种入置有盖玻片的6孔培养板,以不同浓度SST作用72 h,并采用AO/EB荧光染色法[4]测定;流式细胞术:制作HSC悬液并以700 ml/L冷乙醇固定,细胞悬液中加10 g/LRNA酶,37 ℃孵育15 min.再加碘化丙啶1 mL,避光静置30 min后检测凋亡率及细胞周期;TUNEL法:每张盖玻片加TdT,DIG-dUTP1 mL,标记缓冲液18 mL及标记液20 mL,置37℃湿盒中2 h.然后依次加封闭液、Anti-DIG-Biotin及SABC,最终以DAB显色.随机取20个高倍镜视野,计数阳性细胞及凋亡指数;透射电镜检测:以20 ml/L戊二醛固定HSC,离心后包埋、切片,观察超微结构.
统计学处理 采用方差分析及t检验,以SPSS软件分析.
2 结果
2.1 SST对HSC增生的影响 MTT结果显示,SST浓度在10-6-10-9 mol/L范围内均可剂量依赖性地抑制活化的HSC增生,10-6 mol/L及10-7 mol/L组的抑制率分别达30.5 %和15.8 %(P <0.01).
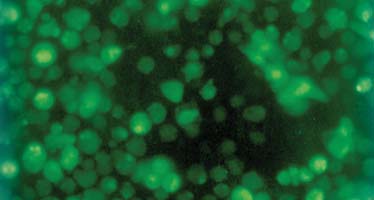
2.2 SST对HSC凋亡及细胞周期的影响 AO/EB荧光染色法:部分HSC变为圆形,核缩小,碎裂,呈颗粒状、块状致密浓染,黄绿色荧光显著增强(图1).计数结果表明,各浓度SST均可促进活化的HSC凋亡,且有明显量效关系,尤以10-6 mol/L及10-7 mol/L组作用最明显(P<0.05).流式细胞术:10-6,10-7,10-8,10-9,10-10mol/L SST处理组及对照组的HSC凋亡率分别为16.6±4.6 %、6.1±1.8%、2.8±0.7%、2.3±1.0 %、1.6±0.6 %及1.5±1.2 %.10-6 mol/L及10-7 mol/L组与对照组相比,凋亡率存在显著性差异(P <0.01).同时,各SST处理组中G0/G1期细胞比例增高,S期细胞比例降低.10-6 mol/L及10-7 mol/L组与对照组比较,G0/G1期细胞由78.5±4.4 %分别提高至93.1±9.7 % (P <0.01)及90.7%±8.0 %(P <0.05),S期细胞则由12.5±2.9 %分别减低至1.8±0.4 % (P <0.01) 及 3.9±0.9 % (P <0.05).TUNEL法:10-6,10-7,10-8,10-9及10-10 mol/L SST处理组的凋亡指数分别为14.7±3.9 %、6.0±1.0 %、2.3±0.6 %、2.0±0.8 %及1.7±0.9 %,其中10-6 mol/L及10-7 mol/L组的凋亡指数明显高于对照组的2.2±0.7 %(P <0.01).加SST 72 h后透射电镜下出现凋亡的形态特征[26]:细胞皱缩,空泡增多,线粒体轻度增多,肿胀,内质网扩张.胞核收缩,染色质凝聚成块,或呈环状致密带,沿核膜内侧排列.并见少量凋亡小体.
图1 SST对活化HSC凋亡的影响 (×400倍)
3 讨论
SST是重要的细胞增生及分化调节肽,通过阻断生长因子及激素的合成、分泌或由受体(SSTR1、SSTR2A、SSTR2B 及SSTR3-SSTR5)介导,可抑制各种细胞增生[5-9],具有广泛的生理作用.SST有SST-14、SST-28等多种分子形式,其中以SST-14最为重要.HSC在肝硬化的发生、发展过程中起关键作用[10-13].近年来发现,肝小叶内的神经纤维含SST,且与肝窦内皮细胞及HSC密切接触[14].2001年Reynaert et al [15]提出,HSC在活化过程中开始表达SSTR1、SSTR2及SSTR3.由此推测,SST可能通过旁泌方式在HSC的活化过程中发挥负调节作用.此外,以自泌和/或旁泌方式释放IGF、EGF及TGF-a等有丝分裂原[10],对HSC的持续激活及肝硬化形成十分重要,而SST可抑制这些细胞因子的分泌,并可逆转EGF对受体的作用.同样提示SST可能具有抗肝硬化作用.
本研究显示,SST浓度在10-6-10-9 mol/L范围内,均可通过剂量依赖方式抑制活化的HSC增生,当浓度达10-6 mol/L或10-7 mol/L时,抑制作用具有统计学意义.SST的增生抑制作用主要由SSTR1、2、4、5介导.通过与G蛋白藕合,SSTR可抑制腺苷酸环化酶活性,使细胞内cAMP水平降低,进而激活PTP,调节MAPK活性,影响c-fos,c-jun,c-myc的转录,最终阻碍细胞增生,并致细胞周期阻滞[16,17].我们发现,经SST处理后G0/G1期细胞比例增高,S期细胞比例降低,这与Sharma et al [16]的报道一致.表明G0/G1期阻滞是SST发挥抗增生作用的重要机制.
活化的HSC主要通过凋亡方式减少[18-22].SST则通过SSTR2、SSTR3诱导细胞凋亡[23-25],其机制与选择性激活酸性核酸内切酶有关.细胞内酸化及酸性核酸内切酶的激活,引起DNA断裂,激活野生型p53.激活的PTP也可使p53丝氨酸残基去磷酸化而活化,从而引起凋亡[27-35].我们运用荧光染色、流式细胞术、TUNEL、透射电镜等方法,从形态及生化角度均证实SST可促进体外原代培养的大鼠HSC凋亡,且SST浓度与HSC凋亡率呈正相关.SST浓度达10-6 mol/L及10-7 mol/L时,凋亡率的改变可达显著性水平.
总之,低剂量SST即可有效抑制活化的HSC增生,提高其凋亡率,因而可能在肝硬化的防治中发挥重要作用,值得进一步深入探讨.
4 参考文献
1 杨利剑.不同生长抑素制剂治疗食管静脉曲张出血31例.世界华人消化杂志 2000; 8(特刊8) : 73
2 龚建平,韩立本.肝脏细胞的分离、培养和鉴定技术.世界华人消化杂志 1999;7:417-419
3 朱永红,胡大荣.肝脏星形细胞系的建立及应用.世界华人消化杂志 1999;7:348-349
4 DL 斯佩克特,RD戈德曼,LA莱因万德,著.细胞实验指南 第1版,北京:科学出版社, 2001:105
5 Melen-Mucha G, Winczyk K, Pawlikowski M. Effects of somatostatin analogs octreotide and lanreotide on the
proliferation and apoptosis in colon 38 tumor: interaction with 5-fluorouracil. Neuroendocrinol Lett 2000;21:137-142
6 Schwab RE, Froidevaux S, Paku S, Tejeda M, Szende B, Pap A, Beglinger C, Eberle AN, Keri G. Antiproliferative efficacy
of the somatostatin analogue TT-232 in human melanoma cells and tumours. Anticancer Res 2001; 21: 71-75
7 Kiaris H, Schally AV, Nagy A, Szepeshazi K, Hebert F, Halmos G. A targeted cytotoxic somatostatin (SST) analogue,
AN-238, inhibits the growth of H-69 small-cell lung carcinoma (SCLC) and H-157 non-SCLC in nude mice. Eur J
Cancer 2001; 37: 620-628
8 Ferjoux G, Bousquet C, Cordelier P, Benali N, Lopez F, Rochaix P, Buscail L, Susini C. Signal transduction of
somatostatin receptors negatively controlling cell proliferation. J Physiol Paris 2000; 94: 205-210
9 Szepeshazi K, Halmos G, Schally AV, Arencibia JM, Groot K, Vadillo-Buenfil M, Rodriguez-Martin E. Growth inhibition
of experimental pancreatic cancers and sustained reduction in epidermal growth factor receptors during therapy with
hormonal peptide analogs. J Cancer Res Clin Oncol 1999; 125: 444-452
10 姜慧卿,张晓岚.肝纤维化的发生机制.世界华人消化杂志 2000;8:687-689
11 蒋树林,姚希贤,孙玉凤.肝纤维化的治疗.世界华人消化杂志 2000;8:684-686
12 白文元,姚希贤,冯丽英.肝纤维化的研究现状.世界华人消化杂志 2000;8:1267-1268
13 王福生,吴祖泽.肝纤维化和肝硬变基因治疗的研究现状.世界华人消化杂志 2000; 8: 371-373
14 Stoyanova II, Gulubova MV. Immunocytochemical study on the liver innervation in patients with cirrhosis.
Acta Hisochem 2000; 102: 391-402
15 Reynaert H, Vaeyens F, Qin H, Hellemans K, Chatterjee N, Winand D, Quartier E, Schuit F, Urbain D, Kumar U, Patel
YC, Geerts A. Somatostatin suppresses endothelin-induced rat hepatic stellate cell contraction via somatostatin
receptor subtype 1. Gastroenterology 2001; 121: 915-930
16 Sharma K, Patel YC, Srikant CB. C-terminal region of human somatostatin receptor 5 is required for induction of Rb
and G1 cell cycle arrest. Mol Endcrinol 1999; 13: 82-90
17 Stetak A, Lankenau A, Vantus T, Csermely P, Ullrich A, Keri G. The antitumor somatostatin analogue TT-232 induces
cell cycle arrest through PKCdelta and c-Src. Biochem Biophys Res Commun 2001; 285: 483-488
18 Issa R, Williams E, Trim N, Kendall T, Arthur MJ, Reichen J, Benyon RC, Iredale JP. Apoptosis of hepatic stellate
cells: involvement in resolution of biliary fibrosis and regulation by soluble growth factors. Gut 2001; 48: 548-557
19 王静艳,刘伟.细胞凋亡与病毒性肝炎.世界华人消化杂志 2002;10:423-425
20 Liu XJ, Yang L, Wu HB, Qiang O, Huang MH, Wang YP. Apoptosis of rat hepatic stellate cells induced by anti-focal
adhesion kinase antibody. World J Gastroenterol 2002; 8: 734-738
21 Yao XX, Tang YW, Yao DM, Xiu HM. Effects of Yigan Decoction on proliferation and apoptosis of hepatic stellate cells.
World J Gastroenterol 2002; 8: 511-514
22 Zhang XL,Liu L,Jiang HQ. Salvia miltiorrhiza monomer IH764-3 induce s hepatic stellate cell apoptosis via caspase-3
activation. World J Gastroenterol 2002; 8: 515-519
23 Teijeiro R, Rios R, Costoya JA, Castro R, Bello JL, Devesa J, Arce VM. Activation of human somatostatin receptor 2
promotes apoptosis through a mechanism that is independent from induction of p53. Cell Physiol Biochem
2002; 12: 31-38
24 Szepeshazi K, Schally AV, Halmos G, Sun B, Hebert F, Csernus B, Nagy A. Targeting of cytotoxic somatostatin analog
AN-238 to somatostatin receptor subtypes 5 and/or 3 in experimental pancreatic cancers. Clin Cancer Res
2001; 7: 2854-2861
25 Rochaix P, Delesque N, Esteve JP, Saint-Laurent N, Voight JJ, Vaysse N, Susini C, Buscail L.Gene therapy for
pancreatic carcinoma: local and distant antitumor effects after somatostatin receptor sst2 gene transfer. Hum
Gene Ther 1999; 10: 995-1008
26 Deng LY, Zhang YH, Zhang HX, Ma CL, Chen ZG. Observation of morphological changes and cytoplasmic movement
in apoptosis process. World J Gastroenterol 1998; 4: 66-67
27 Vantus T, Keri G, Krivickiene Z, Valius M, Stetak A, Keppens S, Csermely P, Bauer PI, Bokonyi G, Declercq W,
Vandenabeele P, Merlevede W, Vandenheede JR. The somatostatin analogue TT-232 induces apoptosis in A431
cells: sustained activation of stress-activated kinases and inhibition of signalling to extracellular signal-regulated kinases.
Cell Signal 2001; 13: 717-725
28 Trobonjaca Z, Radosevic-Stasic B, Crncevic Z, Rukavina D. Modulatory effects of octreotide on anti-CD3
and dexamethasone-induced apoptosis of murine thymocytes. Int Immunopharmacol 2001; 1: 1753-1764
29 Zalatnai A, Szegedi Z, Bocsi J. Flow cytometric evidence of apoptosis in human pancreatic cancer xenografts
treated with Sandostatin (octreotide). Anticancer Res 2000; 20: 1663-1666
30 Liu D, Martino G, Thangaraju M, Sharma M, Halwani F, Shen SH, Patel YC, Srikant CB. Caspase-8-mediated
intracellular acidification precedes mitochondrial dysfunction in somatostatin-induced apoptosis. J Biol Chem
2000; 275: 9244-9250
31 Tompa A, Jakab MG, Major J, Idei M, Bocsi J, Mihalik R, Szende B, Keri G. The somatostatin analogue peptide TT-232
induces apoptosis and chromosome breakage in cultured human lymphocytes. Mutat Res 2000; 465: 61-68
32 Thangaraju M, Sharma K, Leber B, Andrews DW, Shen SH, Srikant CB. Regulation of acidification and apoptosis by
SHP-1 and Bcl-2. J Biol Chem 1999; 274: 29549-29557
33 Thangaraju M, Sharma K, Liu D, Shen SH, Srikant CB. Interdepent regulatio of intracellular acidification and SHP-1 in
apoptosis. Cancer Res 1999; 59: 1649?654
34 刘丽娜.细胞凋亡的分子机制.世界华人消化杂志 2002;10:422-423
35 Szepeshazi K, Schally AV, Halmos G, Armatis P, Hebert F, Sun B, Feil A, Kiaris H, Nagy A. Targeted cytotoxic
somatostatin analogue AN-238 inhibits somatostatin receptor-positive experimental colon cancers independently of
their p53 status. Cancer Res 2002; 62: 781-788
